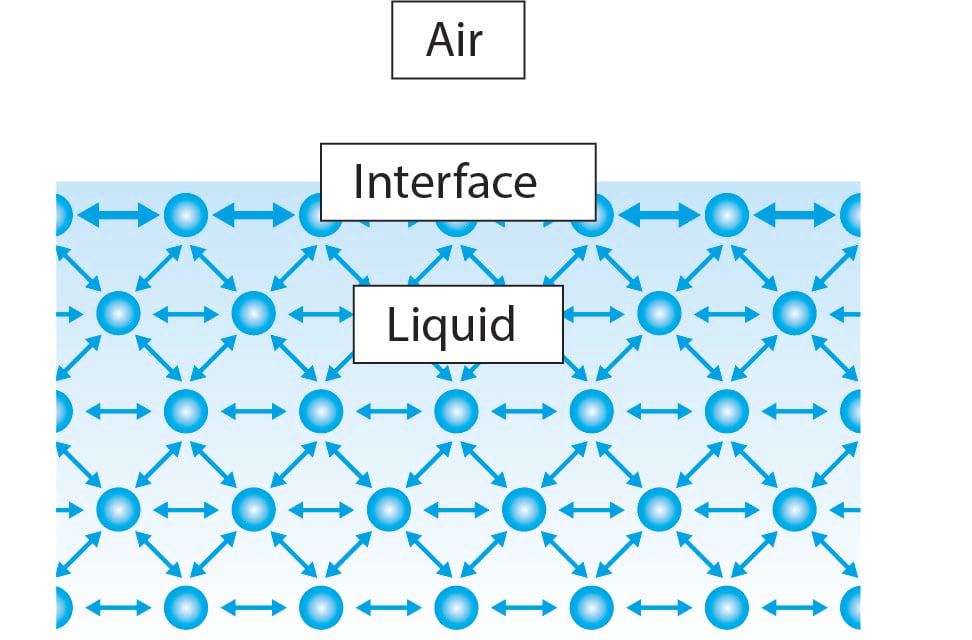
What Causes The High Surface Tension And Low Vapor Pressure Of Water . the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. See examples, diagrams, and practice questions on the. learn about the effects of intermolecular forces on the viscosity, surface tension, and vapor pressure of liquids. learn how cohesion and adhesion affect the behavior of liquids at the interface with air or other liquids. The cohesive forces between liquid molecules. Water also has an exceptionally high heat of. water's high surface tension is due to the hydrogen bonding in water molecules. learn how vapor pressure and surface tension are properties of liquids that depend on temperature and intermolecular forces. learn how water's hydrogen bonding explains its high surface tension and low vapor pressure. surface tension is the tendency of liquid surfaces to shrink into the minimum area possible due to cohesion and adhesion forces.
from www.biolinscientific.com
learn how water's hydrogen bonding explains its high surface tension and low vapor pressure. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. surface tension is the tendency of liquid surfaces to shrink into the minimum area possible due to cohesion and adhesion forces. See examples, diagrams, and practice questions on the. The cohesive forces between liquid molecules. learn how cohesion and adhesion affect the behavior of liquids at the interface with air or other liquids. learn about the effects of intermolecular forces on the viscosity, surface tension, and vapor pressure of liquids. learn how vapor pressure and surface tension are properties of liquids that depend on temperature and intermolecular forces. Water also has an exceptionally high heat of. water's high surface tension is due to the hydrogen bonding in water molecules.
Surface tension of water Why is it so high?
What Causes The High Surface Tension And Low Vapor Pressure Of Water learn how cohesion and adhesion affect the behavior of liquids at the interface with air or other liquids. Water also has an exceptionally high heat of. See examples, diagrams, and practice questions on the. learn how water's hydrogen bonding explains its high surface tension and low vapor pressure. water's high surface tension is due to the hydrogen bonding in water molecules. The cohesive forces between liquid molecules. learn about the effects of intermolecular forces on the viscosity, surface tension, and vapor pressure of liquids. learn how cohesion and adhesion affect the behavior of liquids at the interface with air or other liquids. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. learn how vapor pressure and surface tension are properties of liquids that depend on temperature and intermolecular forces. surface tension is the tendency of liquid surfaces to shrink into the minimum area possible due to cohesion and adhesion forces.
From slideplayer.com
WATER And Solution Formation ppt download What Causes The High Surface Tension And Low Vapor Pressure Of Water surface tension is the tendency of liquid surfaces to shrink into the minimum area possible due to cohesion and adhesion forces. water's high surface tension is due to the hydrogen bonding in water molecules. learn how cohesion and adhesion affect the behavior of liquids at the interface with air or other liquids. the vapor pressure of. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From slideplayer.com
Water and Aqueous Systems ppt download What Causes The High Surface Tension And Low Vapor Pressure Of Water learn how water's hydrogen bonding explains its high surface tension and low vapor pressure. The cohesive forces between liquid molecules. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. See examples, diagrams, and practice questions on the. surface tension is the tendency of liquid surfaces to. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From slideplayer.com
WATER And Solution Formation ppt download What Causes The High Surface Tension And Low Vapor Pressure Of Water Water also has an exceptionally high heat of. See examples, diagrams, and practice questions on the. learn how vapor pressure and surface tension are properties of liquids that depend on temperature and intermolecular forces. surface tension is the tendency of liquid surfaces to shrink into the minimum area possible due to cohesion and adhesion forces. learn how. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From chem.libretexts.org
Chapter 7.3 Unique Properties of Liquids Chemistry LibreTexts What Causes The High Surface Tension And Low Vapor Pressure Of Water learn how water's hydrogen bonding explains its high surface tension and low vapor pressure. The cohesive forces between liquid molecules. learn about the effects of intermolecular forces on the viscosity, surface tension, and vapor pressure of liquids. water's high surface tension is due to the hydrogen bonding in water molecules. See examples, diagrams, and practice questions on. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID What Causes The High Surface Tension And Low Vapor Pressure Of Water Water also has an exceptionally high heat of. learn about the effects of intermolecular forces on the viscosity, surface tension, and vapor pressure of liquids. learn how cohesion and adhesion affect the behavior of liquids at the interface with air or other liquids. learn how water's hydrogen bonding explains its high surface tension and low vapor pressure.. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From www.vrogue.co
A Variation Of The Surface Tension And Viscosity Of T vrogue.co What Causes The High Surface Tension And Low Vapor Pressure Of Water the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. learn how cohesion and adhesion affect the behavior of liquids at the interface with air or other liquids. water's high surface tension is due to the hydrogen bonding in water molecules. The cohesive forces between liquid molecules.. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From www.slideserve.com
PPT Solids and Liquids PowerPoint Presentation, free download ID What Causes The High Surface Tension And Low Vapor Pressure Of Water learn how vapor pressure and surface tension are properties of liquids that depend on temperature and intermolecular forces. The cohesive forces between liquid molecules. learn about the effects of intermolecular forces on the viscosity, surface tension, and vapor pressure of liquids. the vapor pressure of water is the pressure at which the gas phase is in equilibrium. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint What Causes The High Surface Tension And Low Vapor Pressure Of Water learn about the effects of intermolecular forces on the viscosity, surface tension, and vapor pressure of liquids. The cohesive forces between liquid molecules. Water also has an exceptionally high heat of. water's high surface tension is due to the hydrogen bonding in water molecules. learn how cohesion and adhesion affect the behavior of liquids at the interface. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From slideplayer.com
Aqueous Systems The wonder of water. ppt download What Causes The High Surface Tension And Low Vapor Pressure Of Water The cohesive forces between liquid molecules. water's high surface tension is due to the hydrogen bonding in water molecules. surface tension is the tendency of liquid surfaces to shrink into the minimum area possible due to cohesion and adhesion forces. See examples, diagrams, and practice questions on the. learn how vapor pressure and surface tension are properties. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 What Causes The High Surface Tension And Low Vapor Pressure Of Water See examples, diagrams, and practice questions on the. learn how cohesion and adhesion affect the behavior of liquids at the interface with air or other liquids. water's high surface tension is due to the hydrogen bonding in water molecules. learn about the effects of intermolecular forces on the viscosity, surface tension, and vapor pressure of liquids. The. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts What Causes The High Surface Tension And Low Vapor Pressure Of Water learn about the effects of intermolecular forces on the viscosity, surface tension, and vapor pressure of liquids. learn how vapor pressure and surface tension are properties of liquids that depend on temperature and intermolecular forces. The cohesive forces between liquid molecules. the vapor pressure of water is the pressure at which the gas phase is in equilibrium. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From www.askiitians.com
Raoults Law Study Material for IIT JEE askIITians What Causes The High Surface Tension And Low Vapor Pressure Of Water See examples, diagrams, and practice questions on the. learn how vapor pressure and surface tension are properties of liquids that depend on temperature and intermolecular forces. learn how cohesion and adhesion affect the behavior of liquids at the interface with air or other liquids. learn about the effects of intermolecular forces on the viscosity, surface tension, and. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From www.biolinscientific.com
Surface tension of water Why is it so high? What Causes The High Surface Tension And Low Vapor Pressure Of Water Water also has an exceptionally high heat of. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. water's high surface tension is due to the hydrogen bonding in water molecules. See examples, diagrams, and practice questions on the. learn about the effects of intermolecular forces on. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Causes The High Surface Tension And Low Vapor Pressure Of Water Water also has an exceptionally high heat of. learn how vapor pressure and surface tension are properties of liquids that depend on temperature and intermolecular forces. The cohesive forces between liquid molecules. See examples, diagrams, and practice questions on the. learn how cohesion and adhesion affect the behavior of liquids at the interface with air or other liquids.. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From www.slideserve.com
PPT Chapter 17 “Water and Aqueous Systems” PowerPoint Presentation What Causes The High Surface Tension And Low Vapor Pressure Of Water The cohesive forces between liquid molecules. learn how vapor pressure and surface tension are properties of liquids that depend on temperature and intermolecular forces. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. learn how cohesion and adhesion affect the behavior of liquids at the interface. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From www.vrogue.co
Surface Tension Definition Causes Formula Example Emb vrogue.co What Causes The High Surface Tension And Low Vapor Pressure Of Water learn about the effects of intermolecular forces on the viscosity, surface tension, and vapor pressure of liquids. learn how cohesion and adhesion affect the behavior of liquids at the interface with air or other liquids. The cohesive forces between liquid molecules. learn how vapor pressure and surface tension are properties of liquids that depend on temperature and. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From dxopxntvm.blob.core.windows.net
Why Does Water Vapor Pressure Increase With Temperature at Antonio What Causes The High Surface Tension And Low Vapor Pressure Of Water learn how cohesion and adhesion affect the behavior of liquids at the interface with air or other liquids. See examples, diagrams, and practice questions on the. learn how water's hydrogen bonding explains its high surface tension and low vapor pressure. learn about the effects of intermolecular forces on the viscosity, surface tension, and vapor pressure of liquids.. What Causes The High Surface Tension And Low Vapor Pressure Of Water.
From slideplayer.com
Solutions Chapter 15 Chapter ppt download What Causes The High Surface Tension And Low Vapor Pressure Of Water learn how water's hydrogen bonding explains its high surface tension and low vapor pressure. See examples, diagrams, and practice questions on the. the vapor pressure of water is the pressure at which the gas phase is in equilibrium with the liquid phase. learn about the effects of intermolecular forces on the viscosity, surface tension, and vapor pressure. What Causes The High Surface Tension And Low Vapor Pressure Of Water.